Enhancing Clinical Trial Design with AI, Omics, and Multidisciplinary Insights
Date Published: January 9, 2025
The future of clinical trials lies at the intersection of technology and biology. With the advent of artificial intelligence (AI), machine learning (ML), and multi-omics technologies, the design and execution of clinical trials are undergoing a paradigm shift. By addressing inefficiencies and leveraging the power of data, these advancements are transforming trials across diverse fields, from oncology to autoimmune and degenerative diseases. This blog delves into a holistic approach to clinical trial design, integrating these innovations to enhance outcomes.
1. Preplanning Trials with Multidisciplinary Expertise
Preplanning forms the backbone of a successful trial. By integrating electronic health records (EHRs) comprehensively, researchers can bridge data gaps and design robust trials. Molecular biologists, bioinformaticians, and computational biologists contribute critical expertise—designing experimental frameworks, developing algorithms for data integration, ensuring diversity, and incorporating genotype and multi-omics data.
For example, computational biologists play a pivotal role in preprocessing and harmonizing EHR datasets, using AI-driven natural language processing (NLP) tools to extract longitudinal patient information. These efforts streamline participant recruitment and enhance trial monitoring, ensuring that trials are data-driven and inclusive from the outset.
Insight: Tools like HINT and SPOT can further optimize trial design by predicting success probabilities, improving the allocation of resources and focusing on trials with the highest impact potential.
Simplified Takeaway: Leveraging multidisciplinary expertise ensures trials are well-prepared, data-rich, and designed for inclusivity from the beginning.
2. Integrating Genomics, Multi-Omics Data, and Diagnostic Innovations
Multi-omics technologies provide unparalleled insights into patient responses:
- Genomics: Whole-genome sequencing (WGS) identifies genetic markers for treatment, enabling targeted therapies and reducing adverse effects.
- Transcriptomics & Proteomics: Techniques like single-cell RNA-seq uncover cellular dynamics, which are critical for understanding cell therapy mechanisms and optimizing treatment efficacy.
- Metabolomics & Epigenomics: Profiling systemic changes offers deeper mechanistic understanding, such as identifying metabolic shifts that influence therapeutic outcomes in cell therapy.
When combined with AI, these technologies exponentially enhance diagnostics. For instance:
- AI in Radiology: AI-powered tools in radiology, such as convolutional neural networks (CNNs), enhance cancer detection by analyzing MRI and mammography images.
- Deep Learning in Pathology: Deep learning models aid pathology by predicting treatment responses from H&E-stained slides.
Example: SEETrials and CliniDigest can synthesize trial designs and outcomes, enabling researchers to quickly understand and apply lessons from prior studies.
Actionable Insight: Incorporating multi-omics alongside AI ensures a comprehensive approach to identifying patient-specific insights, crucial for precision medicine.
3. Addressing Omics Disparities
Current data reveal significant gaps in multi-omics integration across trials. For example:
- Cancer Studies: Cancer studies more frequently include genotype data than infectious diseases.
- Underrepresented Datasets: Limited large-scale studies integrate transcriptomics, genotype, and proteomics simultaneously.
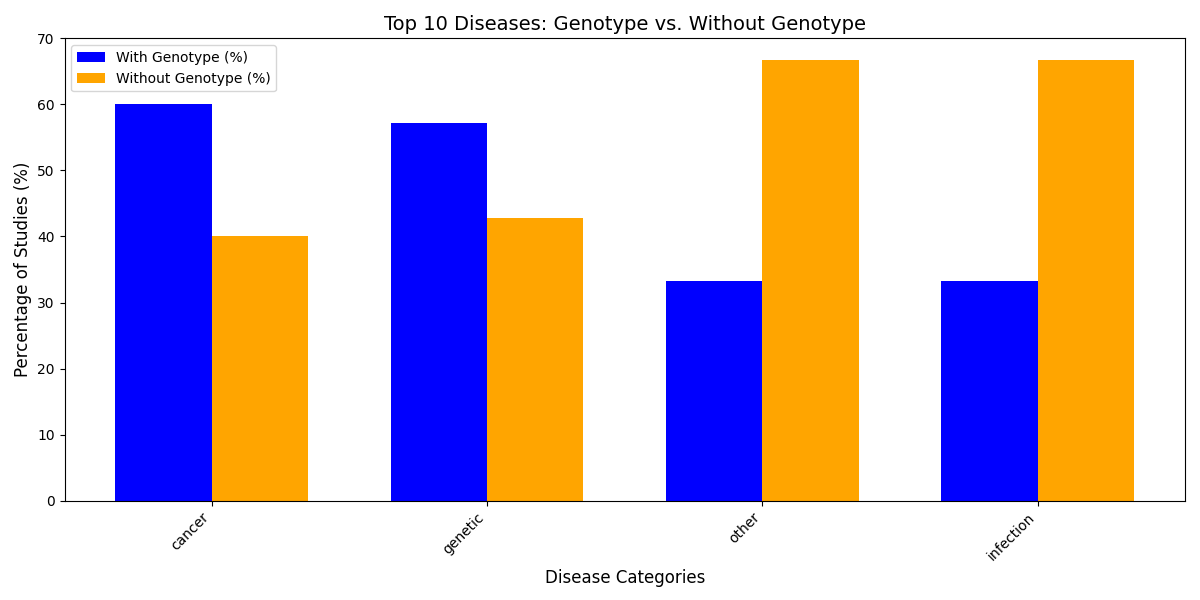
Figure 1: Genotype data inclusion across disease categories, using infectious disease and cancer as examples.
4. Distribution of Omics Type Combinations in Studies
A detailed analysis of omics type combinations in the top 20 large-scale studies highlights critical gaps in data integration. For example:
- Genotype Data Dominance: A majority of studies focused exclusively on genotype data, with limited incorporation of transcriptomics and proteomics.
- Underrepresentation of Multi-Omics: Comprehensive datasets combining genotype, proteomics, and transcriptomics are significantly underrepresented, hindering deeper insights into patient variability and therapeutic outcomes.
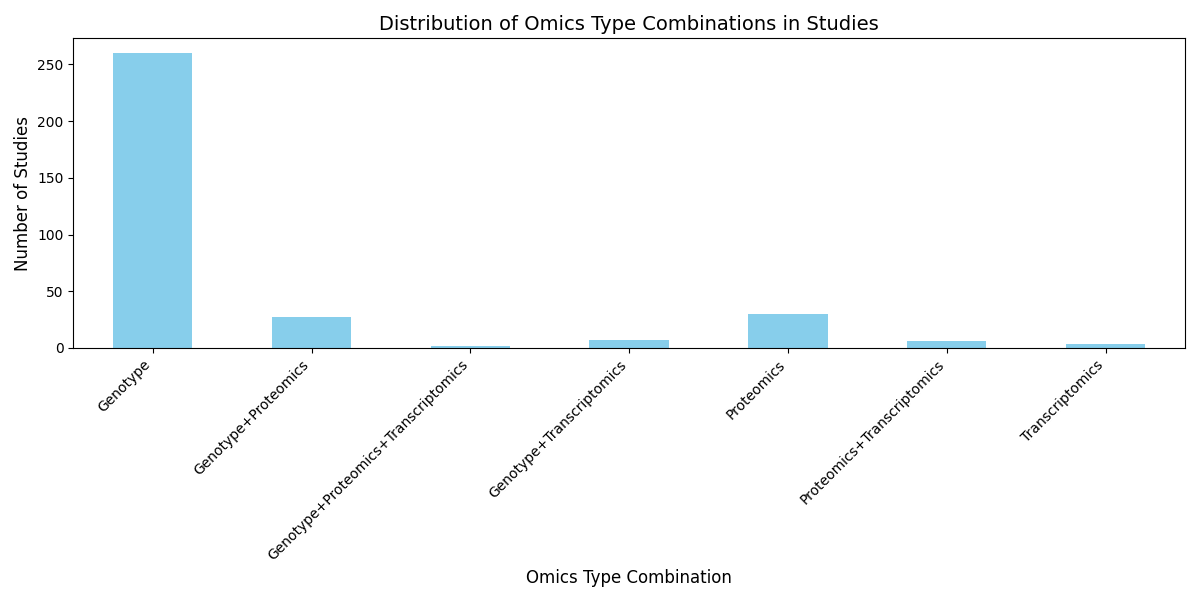
Figure 2: Distribution of omics type combinations in large-scale studies, highlighting the dominance of genotype data and underrepresentation of multi-omics approaches.
5. Addressing Controls and Bias
Traditional controls, while effective, often have limitations. AI introduces innovative solutions:
- Placebo and Active Comparators: Maintain robust traditional controls but integrate digital twins to predict outcomes and enhance statistical power.
- Bias Reduction: AI ensures diverse participant recruitment by analyzing demographic and socioeconomic data.
Operational biases, such as variability in surgical techniques, can also significantly impact outcomes. To mitigate these biases:
- Standardized Operating Protocols: Develop detailed guidelines and provide comprehensive training to ensure uniform techniques across all sites.
- Robotic-Assisted Procedures: Where feasible, implement robotic systems to perform critical tasks with precision, minimizing variability introduced by human factors.
- Real-Time Monitoring and Feedback: Utilize imaging tools and intraoperative sensors to monitor procedural accuracy, providing immediate feedback to operators.
- AI-Powered Quality Control: Leverage machine learning algorithms to analyze procedural data and flag deviations from the standardized protocol.
By addressing both traditional biases and operational inconsistencies, clinical trials can achieve more reliable and reproducible results.
6. Ethical Considerations and AI Challenges
- Biases in Training Data: AI models trained on non-diverse datasets risk perpetuating disparities.
- Explainability Issues: Stakeholders demand transparency in decision-making, but AI’s "black box" nature complicates this.
Insight: Digital twin models from companies like Unlearn can mitigate biases by simulating diverse patient populations, reducing reliance on traditional control groups and improving representativeness.
Actionable Insight: Develop explainable AI frameworks and prioritize diverse, representative datasets to enhance trust and equity.
Conclusion
By integrating AI, omics technologies, and multidisciplinary insights, clinical trials can address inefficiencies, enhance precision, and adopt patient-centric approaches. Computational biologists and cell therapy researchers are uniquely positioned to collaborate in designing data-driven trials, leveraging omics data to stratify patients and optimize treatments. As these innovations mature, they promise to redefine clinical research, fostering equitable and impactful outcomes for patients worldwide.